Fertility startup Inito has secured $29 million in Series B funding to expand its at-home health diagnostics platform and leverage artificial intelligence (AI) in the design of antibodies for new testing capabilities. The company aims to make comprehensive hormone testing accessible outside of clinical settings, offering a more convenient and data-rich approach to personal health monitoring.
Launched in 2021, Inito initially focused on providing quantitative fertility hormone testing for consumers. Now, the company is pivoting towards a broader range of at-home diagnostics, fueled by its latest investment and advancements in AI-driven antibody development.
Expanding At-Home Hormone Testing with AI
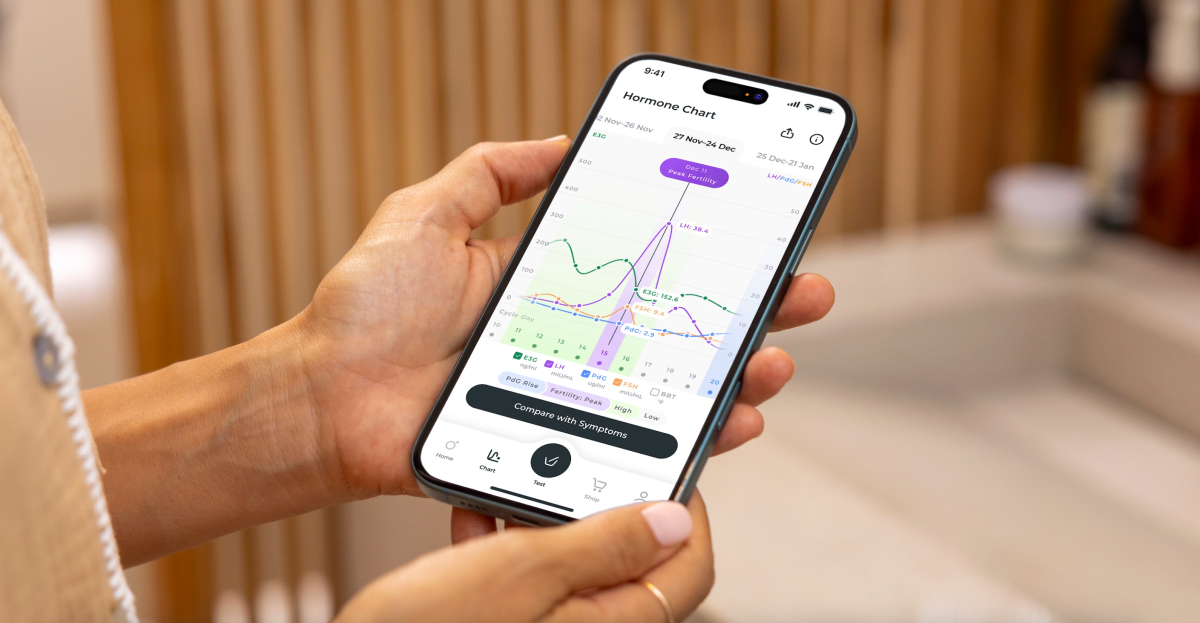
Traditional at-home ovulation tests typically measure estrogen and luteinizing hormone (LH) to predict fertile windows. However, these tests don’t directly confirm ovulation by measuring progesterone metabolite PdG. Inito’s device allows users to measure estrogen, LH, PdG, and follicle-stimulating hormone (FSH) using a single test strip. The company’s AI algorithms then analyze these hormone levels to identify fertility patterns, pinpoint fertile days, and confirm ovulation.
Since its launch, Inito has analyzed over 30 million fertility hormone data points, establishing a substantial dataset for its AI models. This data is crucial for refining the accuracy and insights provided by the platform. The company believes that healthcare should be more accessible and proactive, starting with at-home diagnostics.
The Role of AI-Designed Antibodies
A key component of Inito’s expansion strategy is its investment in AI-engineered antibodies. Traditional antibody development involves growing antibodies in animals and manually screening them in a lab – a process that is both time-consuming and expensive. Furthermore, conventional antibodies often lack the sensitivity required for accurate at-home testing of many biomarkers.
Inito’s approach utilizes AI to predict protein folding, design synthetic antibodies, and virtually test millions of variants before physical production. According to Inito co-founder and CTO Varun Venkatesan, this process yields antibodies that are more sensitive, consistent, and stable than those created through traditional methods. This technology is currently in research and development, with promising initial results from wet lab testing.
This innovation is expected to unlock a new generation of at-home tests, going beyond fertility tracking. The company is exploring applications in areas like pregnancy monitoring, menopause management, and broader endocrine health assessments.
Beyond Fertility: A Broader Vision for At-Home Diagnostics
Inito’s long-term goal is to create a comprehensive at-home health diagnostics platform. CEO Aayush Rai envisions a future where individuals can routinely monitor their hormone levels and gain insights into their overall health without relying solely on clinic visits and lab schedules. This includes expanding the range of measurable biomarkers and providing personalized interpretations of the data.
The company plans to use the new funding to scale manufacturing capabilities and expand its global reach, particularly in the United States and new international markets. Demand for at-home health solutions, including fertility tracking, continues to grow as consumers seek greater control over their healthcare.
The Series B funding round was led by Bertelsmann India Investments and Fireside Ventures, bringing Inito’s total funding to approximately $45 million. Previous investors include Fireside Ventures, Y Combinator, former Nurx CEO Varsha Rao, and various physicians and family offices. The increasing investment in the digital health sector reflects a broader trend towards preventative and personalized medicine.
Looking ahead, Inito aims to launch new tests powered by its AI-designed antibodies and expand its platform’s capabilities. The company’s success will depend on its ability to maintain the accuracy and reliability of its tests while scaling production and navigating regulatory hurdles. The timeline for the release of these new tests remains uncertain, but the company anticipates further updates in the coming months regarding its progress in hormone testing and platform development.